Difference between revisions of "NMN"
(Created page with "{{Infobox | title = Nicotinamide mononucleotide | image = 200px|alt=Nicotinamide mononucleotide | caption1 = {{{caption|}}} <!-- Clinical data --> | dat...") |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 31: | Line 31: | ||
}} | }} | ||
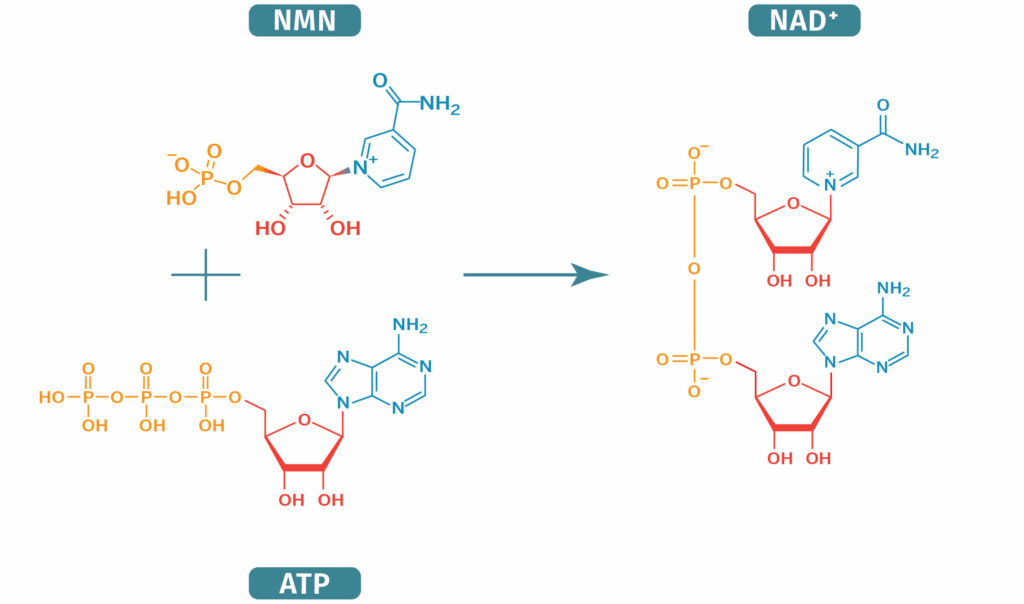
| − | <onlyinclude>{{PageTitle|Nicotinamide mononucleotide|link=NMN}} ('''NMN, NAMN, and β-NMN''') is a [[wikipedia:Nucleotide|nucleotide]] derived from ribose and nicotinamide. | + | <onlyinclude>{{PageTitle|Nicotinamide mononucleotide|link=NMN}} ('''NMN, NAMN, and β-NMN''') is a [[wikipedia:Nucleotide|nucleotide]] derived from ribose and nicotinamide. [https://www.nmn.com/precursors/what-is-nmn NMN] is a precursor of [[NAD+ | nicotinamide adenine dinucleotide (NAD+)]], a form of niacin, also known as [[vitamin B3|vitamin B<sub>3</sub>]]<ref name="imai2013"> Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes, Obes Metab. 2013;15(S3):26-33.</ref>. Because it is a source of cellular energy due to its role in the production of NADH/NAD+, NMN is involved in numerous cellular reactions. Inside the mitochondria, NADH is converted to NAD+ in the process of oxidative phosphorylation. NAD plays a critical role in the TCA cycle, by alternately accepting and donating an electron at various steps of the cycle. NAD+ also plays a key role in regulating enzymes called [[sirtuins]] that play an important role in DNA repair. Numerous studies, mostly done in mice and roundworms, have focused on the potential health benefits of NMN. Formally, NMN may also be known as ‘Nicotinamide D-ribonucleotide’ or ‘β-Nicotinamide ribose monophosphate’ and has the chemical formula C<sub>11</sub>H<sub>15</sub>N<sub>2</sub>O<sub>8</sub>P<ref name="nationalcenterforbiotechnologyinformation"> National Center for Biotechnology Information. [(2S,3S,4R,5R)-5-(3-Carbamoylpyridin-1-ium-1-yl)-3,4-dimethyloxolan-2-yl]methyl hydrogen phosphate | C13H19N2O6P - PubChem. PubChem Database. </ref>. It occurs naturally in small amounts in [[Dietary sources of NMN|dietary sources]] such as cabbage, avocado, and broccoli. |
</onlyinclude> | </onlyinclude> | ||
| Line 166: | Line 166: | ||
==References== | ==References== | ||
<references /> | <references /> | ||
| + | [[Category:Compounds]] | ||
Latest revision as of 23:48, 18 June 2020
 | |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
Nicotinamide mononucleotide (NMN, NAMN, and β-NMN) is a nucleotide derived from ribose and nicotinamide. NMN is a precursor of nicotinamide adenine dinucleotide (NAD+), a form of niacin, also known as vitamin B3[1]. Because it is a source of cellular energy due to its role in the production of NADH/NAD+, NMN is involved in numerous cellular reactions. Inside the mitochondria, NADH is converted to NAD+ in the process of oxidative phosphorylation. NAD plays a critical role in the TCA cycle, by alternately accepting and donating an electron at various steps of the cycle. NAD+ also plays a key role in regulating enzymes called sirtuins that play an important role in DNA repair. Numerous studies, mostly done in mice and roundworms, have focused on the potential health benefits of NMN. Formally, NMN may also be known as ‘Nicotinamide D-ribonucleotide’ or ‘β-Nicotinamide ribose monophosphate’ and has the chemical formula C11H15N2O8P[2]. It occurs naturally in small amounts in dietary sources such as cabbage, avocado, and broccoli.
Contents
History
NMN first gained notoriety in 1963, when Chambon, Weill, and Mandell reported that the molecule activated a newly-discovered DNA-dependent polyadenylic acid synthesizing nuclear enzyme. This led to a series of discoveries concerning nuclear enzymes called poly-ADP-ribose and poly-ADP-ribose polymerases (PARPs). Further work throughout the 1960s helped scientists to understand the biosynthetic pathway that connected niacin, nicotinamide, and NMN[3]. Through this initial research, scientists came to understand the vital role that NMN and NAD+ played in cellular metabolism and oxidation-reduction reactions. Following this, renewed interest in NMN and NAD+ came in the 2000s, when researchers discovered that these compounds were linked to sirtuins, a class of enzymes, whose DNA repair activity plays an active role in aging.
Structure
NMN is a nucleotide product of a nucleoside, composed of ribose and nicotinamide, that reacts with a phosphate group. The chemical formula for NMN is C11H15N2O8P, and the nucleotide exists as a combination of two anomers, of which the beta-anomer is the biologically active form[2].
Mechanism of action
- Main article: NAD+
NMN is the immediate precursor to NAD+, a compound with myriad reported biological activities. Previous research has shown that NMN functions primarily as an intermediate, with few direct mechanisms of action[4]. Instead, it has been shown that NMN supplementation or administration leads to an increase in the measured levels of NAD+[4].
Mechanism of NAD+
Cellular metabolism
NAD+ is a crucial cofactor, which is fundamental to metabolism in humans and many other organisms. NAD+ is a dinucleotide, and its primary mechanism of action in cells is to function as a cofactor in reactions involving cellular metabolism. In its primary function as an electron-accepting molecule, NAD+ transfers electrons between other molecules in biochemical reactions. It plays a central role in cellular reduction-oxidation reactions such as the breakdown of glucose and fatty acids, allowing these substances to be utilized as energy sources[5].
Cell signaling
In addition to its central role in metabolism, NAD+ is also involved in a process known as ADP-ribosylation[6]. This process consists of the addition of one or more ADP-ribose groups to mature proteins. Protein ribosylation has been demonstrated to play a role in DNA repair processes as well as the modification of telomeres – both functions have well-established roles in the progression of cellular aging[7].
NAD+ has also been identified as an extracellular messenger. Preliminary research has highlighted the potential role of NAD+ in signaling between neurons, blood vessels, and even its potential role as a signaling molecule to allow for communication between nerves and the muscles they innervate[8][9].
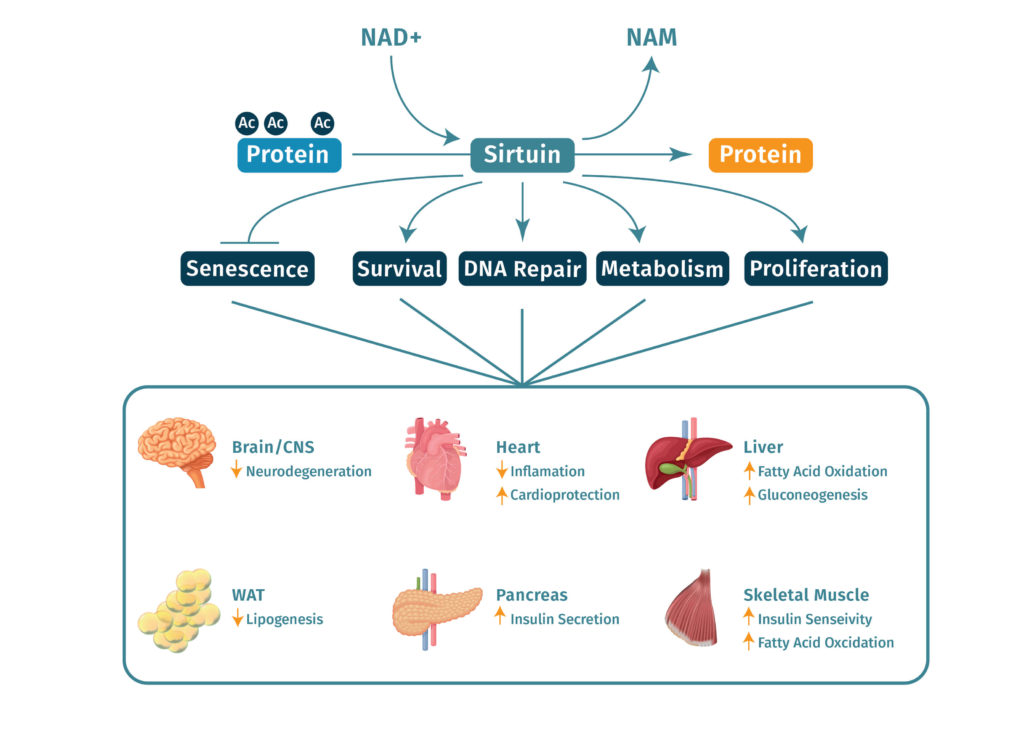
NAD+ and sirtuins
Another potentially relevant mechanism of action of NAD+ is that of its role as a cofactor for sirtuins. Sirtuins are enzymes that act in mitochondrial function and cellular aging. Because NAD+ is considered the rate-limiting substrate for reactions involving sirtuins, significant attention has been placed on modulating its levels to influence the downstream effects through sirtuin-mediated reactions[1][10][11].
Usage
- Main article: NMN usage
NMN is present in several nutritional sources including avocados, cabbage, broccoli, and tomato. The total concentration from these food sources ranges from 0.25 – 1.5 mg NMN / 100 g food source[12]. Once ingested, NMN is absorbed into the circulation. Recently, researchers identified a transporter which is crucial for intestinal absorption of NMN in mice, Slc12a8[12]. They showed that this transporter is specific for NMN and does not transport NAD+ or other precursors of nicotinic acid. Although this transporter may play a role in the human absorption of NMN as well, the corresponding human studies have not yet been carried out.
Bioavailability
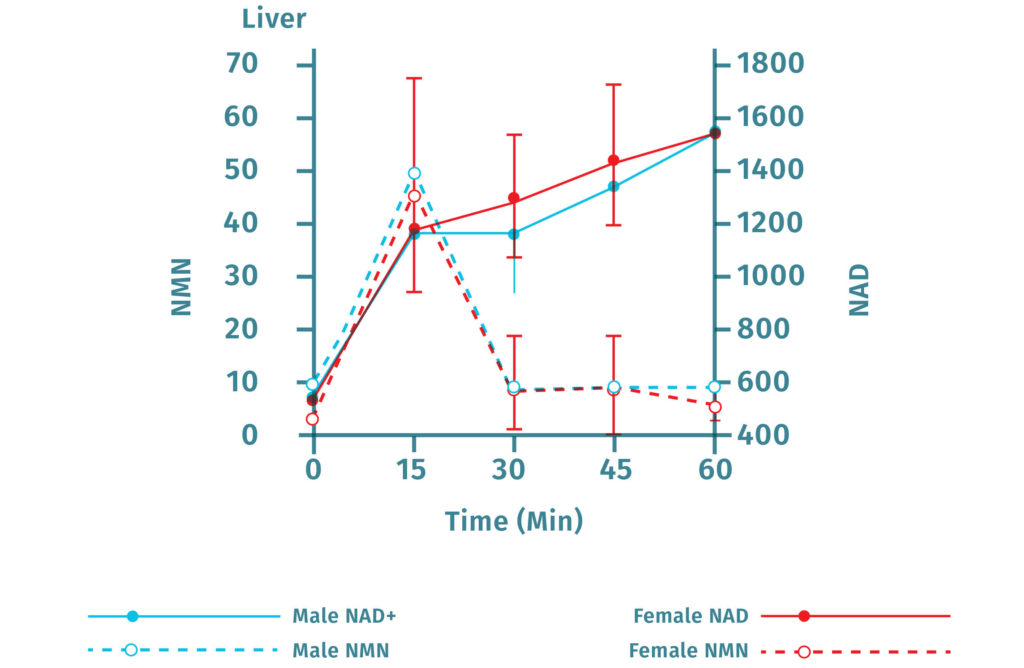
Several studies have investigated the bioavailability of NMN in animal models[4][12][13]. One study of NMN pharmacokinetics in mice found that plasma levels of NMN increased significantly around 2-3 minutes following oral administration. Following this, plasma levels continued to increase through the following 5 - 10 minutes and then returned to baseline by 15 minutes[12]. Further investigation into the bioavailability of NMN using radiolabeled NMN showed that after initial absorption, NMN is quickly converted into biologically active NAD+, which then is rapidly transported to end-effect tissue such as muscle. This study revealed that at 10 minutes post administration, the radiolabeled NMN was only present in the liver and not muscle, but that by 30 minutes the concentrations of peripheral NAD+ had increased while the liver concentration had decreased[12].
Little research has so far been done to examine the bioavailability or pharmacokinetics of NMN in humans. One recent study examined the effects of NMN supplementation in 10 healthy adult volunteers. The researchers administered single oral doses of between 100mg to 500mg NMN. The researchers were not able to directly measure NMN levels in the blood (likely due to sample processing error), but they were able to show dose-dependent increases of two key NMN metabolites from administration through 300 minutes post administration.
Supplementation
Although NMN is found in small quantities in food sources, there is significant interest in supplementing NMN intake to boost its potential positive effects. Animal models have demonstrated improvements in several outcomes related to increased NMN intake, such as metabolism, insulin sensitivity and suppression of age-related weight gain[4][12]. Although human supplements currently exist, little research has been done investigating the efficacy of human NMN supplementation.
Medical uses
NMN has been used as a so-called ‘nutraceutical’. Nutraceuticals are a class of supplement, which are essentially foods or compounds other than synthetic pharmaceuticals which are used for a supposed medicinal purpose. NMN’s purported role as a nutraceutical is based on significant animal research that demonstrated that supplementation was associated with increased longevity, primarily by fighting the age-related decline of the cell’s energy production and mitochondrial functioning. NMN has also been shown to be useful for reducing insulin resistance (the underlying problem in the most common form of a diabetes) in mice[12].
Effects
- See also: Health
NMN has been shown to have a number of downstream effects in several animal-based studies.
- Decreased insulin resistance
Insulin resistance is a medically important condition, as this is the primary problem in type 2 or adult-onset diabetes, the most common form of diabetes. NMN supplementation has been shown to improve insulin resistance and promote insulin sensitivity via previously described mechanisms involving sirtuins, as well as by increasing the overall rate of NAD+ biosynthesis[4][14].
- Improved mitochondrial function
The mitochondria are the energy centers of cells and their overall decline with age is thought to be one of the primary mechanisms through which aging exerts its negative effects. Several studies have demonstrated the relationship between NMN supplementation and improved mitochondrial function in several different tissue types including skeletal muscle, the eye, and even blood vessels[15][16][17].
- Reduction of age-related DNA damage
Aging has been linked with DNA damage as well as premature telomere shortening. Previously studies have established the relationship between the effects of NMM and NAD+ on ADP-ribosylation, as they relate to DNA damage and telomere modification[7]. A recent study of NMN supplementation in mice found that NMN stabilized telomere length while reducing markers associated with DNA damage and improving markers related to mitochondrial functioning and liver damage[18].
- Rescue of age-related decline in female fertility
Additional research has also highlighted the potential effects of NMN supplementation on female oocyte viability and health. A recent animal study, published in the journal Cell Reports, showed that loss of oocyte quality with age was an NAD+ dependent process[19]. The authors found that when they supplemented the animals with NMN, there was an increase in oocyte quality in aged female animals. The results also demonstrated that this improved quality was transferred to the resultant embryos, where NMN supplementation reversed the age-associated adverse effects on embryo viability and development. Based on these results, the authors concluded that NMN supplementation may offer an avenue to reverse age-related declines in female fertility in humans, although further studies are needed.
Research
- See also: NMN research
The studies that led to the recognition of NMN and NAD+ as biologically relevant compounds took place in the first half of the twentieth century. This initial research elucidated the role that these compounds played in reduction-oxidation reactions in cells and the importance of this pathway for diseases involving metabolism such as pellagra (a deficiency of nicotinic acid). This first ‘era’ of NMN studies helped researchers to understand the vital role that NMN plays in helping to promote cellular energy production, especially within the mitochondria.
More recently, in the 2000s, renewed interest in NMN began after scientists discovered the role that this compound plays in interacting with sirtuins, which are important for aging and mitochondrial functioning. This revitalization of interest in NMN and NAD+ has resulted in several animal studies which have reported on the potential benefits of NMN supplementation including: improved insulin sensitivity, improved mitochondrial functioning, and even a decrease in neuronal cell death in Alzheimer’s animal models[12][16][20].
Given the significant results that have been observed after NMN supplementation in animals, attention has turned to the potential effects of human supplementation. Recently, a study evaluating human NMN dosing concluded, demonstrating safety of several doses of oral NMN including 100 mg, 250 mg, and 500 mg doses19. After this first pilot study verifying the safety of oral NMN dosing in humans, further research studies attempting to verify beneficial effects in human trials are likely forthcoming.
Some key figures in NMN research today include David Sinclair and Shin-ichiro Imai.
Synthesis
Biosynthesis
NMN is an intermediate in the eventual production of NAD+, which is the predominant biologically active compound. NAD+ can be synthesized biologically via three mechanisms. However, NMN is only involved in two of these pathways. The two pathways are 1) the nicotinamide salvage pathway and 2) the nicotinamide riboside (NR) pathway.
Nicotinamide salvage pathway
This pathway relies on the biological salvage of the nicotinamide compound. This is the most utilized pathway in mammalian cells and makes use of the byproducts of NAD+ breakdown[21]. In this pathway, the enzyme nicotinamide phosphoribosyltransferase catalyzes the transfer of a phosphoribosyl group from phosphoribosylpyrophosphate to nicotinamide to form NMN.
Nicotinamide riboside pathway
This pathway is less commonly utilized than the corresponding salvage pathway. Here, NMN is formed via the phosphorylation of NR by nicotinamide riboside kinase[22].
In vitro synthesis
The literature surrounding industrial synthetic production of NMN is currently sparse. One recent investigation showed a ‘proof of concept’ by utilizing Escherichia coli[23]. The group reported that by utilizing plasmids that incorporated nicotinamide phosphoribosyl transferase, nicotinamide, and phosphoribosyl pyrophosphate synthetase, they were able to produce NMN at a yield of about 15 mg per 1 L of bacterial culture.
Distribution
- Main article: NMN Products
While the research concerning NMN and its potential benefits is ongoing, several companies have developed commercial products containing NMN.
Notable users
Notable users of NMN include:
David Sinclair is probably the best-known user of NMN. A professor of genetics at Harvard Medical School, Dr. Sinclair reportedly takes 1g of NMN daily, among other supplements.
Joe Rogan reportedly began taking NMN supplements after he interviewed David Sinclair on his podcast in 2019.
Legality
- Main article: Legality of NMN
United States - NMN is legal as a dietary supplement, but without a designation as “Generally Regarded as Safe”.
United Kingdom - NMN containing products are currently available for sale within the UK.
Canada - Currently NMN is not available for sale in Canada. No company has been issued a Natural Products Number which would permit the sale of NMN in Canada.
Japan - NMN containing products are currently available for sale within Japan.
China - Several NMN containing supplements are currently available for sale in China.
Australia - NMN is currently available for sale in Australia.
References
- ↑ 1.0 1.1 Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes, Obes Metab. 2013;15(S3):26-33.
- ↑ 2.0 2.1 National Center for Biotechnology Information. [(2S,3S,4R,5R)-5-(3-Carbamoylpyridin-1-ium-1-yl)-3,4-dimethyloxolan-2-yl]methyl hydrogen phosphate | C13H19N2O6P - PubChem. PubChem Database.
- ↑ IKEDA M, TSUJI H, NAKAMURA S, ICHIYAMA A, NISHIZUKA Y, HAYAISHI O. STUDIES ON THE BIOSYNTHESIS OF NICOTINAMIDE ADENINE DINUCLEOTIDE. II. A ROLE OF PICOLINIC CARBOXYLASE IN THE BIOSYNTHESIS OF NICOTINAMIDE ADENINE DINUCLEOTIDE FROM TRYPTOPHAN IN MAMMALS. J Biol Chem. 1965;240:1395-1401.
- ↑ 4.0 4.1 4.2 4.3 4.4 Yoshino J, Mills KF, Yoon MJ, Imai SI. Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528-536.
- ↑ Cantó C, Menzies KJ, Auwerx J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31-53.
- ↑ Ziegler M. New functions of a long-known molecule: Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267(6):1550-1564.
- ↑ 7.0 7.1 Bürkle A. Poly(ADP-ribose): The most elaborate metabolite of NAD+. FEBS J. 2005;272(18):4576-4589.
- ↑ Mutafova-Yambolieva VN, Sung JH, Hao X, et al. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104(41):16359-16364.
- ↑ Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of β-nicotinamide adenine dinucleotide upon stimulation of postganglionic nerve terminals in blood vessels and urinary bladder. J Biol Chem. 2004;279(47):48893-48903.
- ↑ Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130(8):518-527.
- ↑ Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: Attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol - Hear Circ Physiol. 2008;294(6):H2721-35.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 Mills KF, Yoshida S, Stein LR, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24(6):795-806.
- ↑ Grozio A, Mills KF, Yoshino J, et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab. 2019;1(1):47-57.
- ↑ Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia. 2011;54(12):3083-3092.
- ↑ Uddin GM, Youngson NA, Sinclair DA, Morris MJ. Head to head comparison of short-term treatment with the NAD+ precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front Pharmacol. 2016;7(AUG).
- ↑ 16.0 16.1 Tarantini S, Valcarcel-Ares MN, Toth P, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24.
- ↑ Lin JB, Kubota S, Ban N, et al. NAMPT-Mediated NAD+ Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016;17(1):69-85.
- ↑ Amano H, Chaudhury A, Rodriguez-Aguayo C, et al. Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab. 2019;29(6):1274-1290.e9.
- ↑ Michael Bertoldo AJ, Listijono DR, Jonathan Ho W-H, Sinclair DA, Homer HA, Wu LE. NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 2020;30.
- ↑ Wang X, Hu X, Yang Y, Takata T, Sakurai T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1-9.
- ↑ Venter G, Oerlemans FTJJ, Willemse M, Wijers M, Fransen JAM, Wieringa B. NAMPT-Mediated Salvage Synthesis of NAD+ Controls Morphofunctional Changes of Macrophages. Dzeja P, ed. PLoS One. 2014;9(5):e97378.
- ↑ Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495-502.
- ↑ Marinescu GC, Popescu RG, Stoian G, Dinischiotu A. β-nicotinamide mononucleotide (NMN) production in Escherichia coli. Sci Rep. 2018;8(1):1-11.