NAD+
 | |||||||||
| |||||||||
| |||||||||
Nicotinamide adenine dinucleotide (NAD+) is a co-enzyme, syntesized from vitamin B3, found in all living cells. NAD+ is one of the most abundant molecules in our body, involving in over 500 enzymatic reactions.[1] The molecule supports the highest number of biochemical reactions next only to water.[2] Nicotinamide mononucleotide (NMN), niacin, nicotinamide (NAM), and nicotinamide riboside (NR) all are vitamin precursors of NAD+.[3] NAD+ is known as a ‘helper’ molecule which is involved in a choreography of reactions which lead to its binding to proteins and as a result activation of enzymes which initiate signaling pathways throughout the body. Evolving evidence highlights boosting levels of NAD+ as playing a pivotal role in reducing the signs of aging and age-related disease.[4] NAD+ has been shown to decline with age due to a reduced ability of the cells to recycle or synthesize NAD+. NAD+ regulates protein-protein interactions involved with DNA repair[5]
Contents
History
1906 NAD was discovered by British biochemists, Arthur Harden and William John Young [6]. Initially Louis Pasteur recognized that yeast cells were responsible for fermentation[7] and Arthur Harden was intrigued to learn more about the details of this process. As a result, Harden and Young were able to separate the yeast cells into two fractions, heat stable and heat labile. The heat labile fraction contained proteins required for fermentation whilst the heat stable fraction contained co-factors such as NAD+ that ‘helped’ the proteins perform such functions.
1929 Hans von Euler-Chelpin furthered the work of Harden and Young by extensively separating the components of the heat-stable fraction of yeast cells, which allowed him to obtain a purified form of nucleotide sugar phosphate (NAD) as well as to determine the chemical structure. [7][8]
1936 Otto Heinrich Warburg demonstrated NAD’s role in fermentation reactions. He found that the nicotinamide portion of NAD was required for a hydride transfer reaction to occur.[7]
1938 Conrad Elvehjam discovered that nicotinic acid extracted from fresh liver was able to cure black tongue (pellagra) in canines.[7][9]
1940 Arthur Kornberg studied the synthesis of NAD in the body using advanced purification of proteins and co-enzymes. [7]
1958 Jack Priess and Philip Handler uncovered the conversion of nicotinic acid to NAD via a three-step pathway which became known as the Priess-Handler pathway.[7]
1963 Mandel and colleagues identified a chemical reaction that broke NAD into two parts, nicotinamide and ADP-ribose.[7][10]
2000 Leonard Guarente and co-workers discovered sirtuin enzymes capable of expanding the lifespan of yeast using NAD to help keep genes in a ‘silent’ or non-functional mode. [7][11]
2004 Charles Brenner and colleagues uncovered a two-step kinase pathway in which nicotinamide riboside was converted to NAD+.[3]
Structure
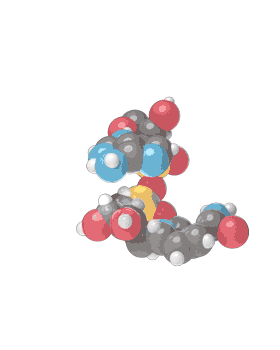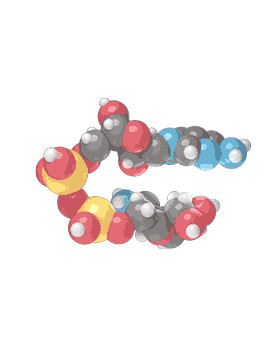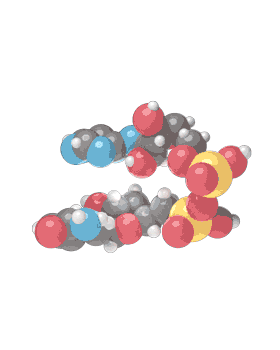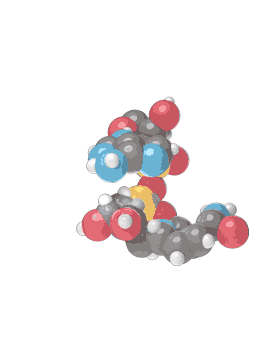
Molecular formula C21H27N7O14P2 [12] Consists of 2 nucleosides (one joined to an adenine nucleobase and the other to nicotinamide) bridged by a phosphate group. They contain a ribose ring, one with adenine attached to the first carbon atom and one with nicotinamide at the same position. The structure exists as diastereomers meaning the nicotinamide moiety can be attached in two different orientations.
NAD+ is an oxidizing agent and is involved in electron transfer reactions in which it accepts electrons form other molecules to become reduced, hence forming NADH which as a reducing agent is then able to donate electrons. NAD+ (the plus sign represents the formal charge on one of its nitrogen atoms) is known as nicotinamide adenine dinucleotide in the oxidized form, with NADH being the reduced form.
Biosythesis
There are 2 ways by which NAD+ is synthesized. It can be produced from amino acids in the de novo pathway or by recycling components such as nicotinamide back to NAD+, in what is called the salvage pathway.
Quinolinic acid
Quinolinic acid (QA) is generated from an amino acids such as tryptophans in animals or aspartic acid in bacteria or plants. QA is then converted to nicotinic acid mononucleotide (NaMN) via transfer of a phosphoribose group. An adenylate group is then transferred to form NaAD. Finally, the nicotinic acid group undergoes amidation to form nicotinamide (Nam), hence resulting in nicotinamide adenine dinucleotide.[13]
Salvage pathway
Some cells salvage preformed compounds that contain a pyridine base. This requires the three vitamin precursors, nicotinic acid (NA), nicotinamide (NAM) and nicotinamide riboside (NR). Such precursors termed vitamin B3 can be absorbed by the body through the usual dietary intake.
Functions
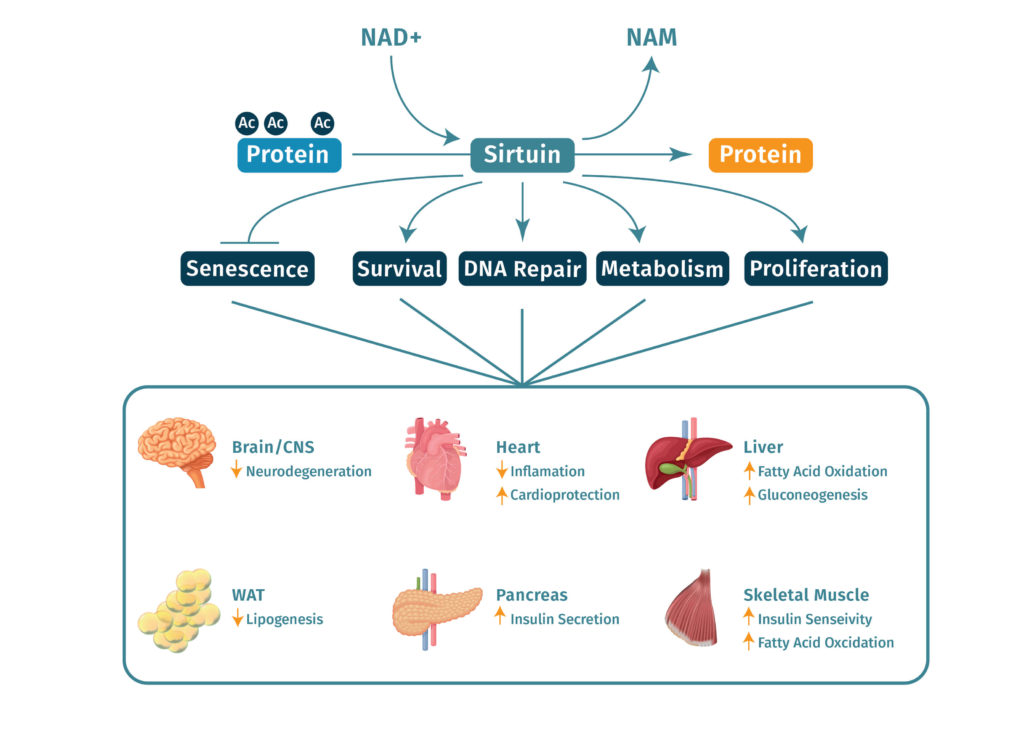
NAD+ is vital for the creation of energy and the regulation of cellular processes within humans, mammals, bacteria and even plants. This critical co-enzyme is involved in an array of metabolic pathways, namely converting nutrients into energy or working as a helper molecule for proteins.
Cellular Metabolism
NAD+ is a crucial cofactor which is fundamental to metabolism in humans and many other organisms. NAD+ is a dinucleotide, and its primary mechanism of action in cells is to function as a cofactor in the reactions involved in cellular metabolism. NAD+ primarily acts as an electron accepting molecule, which then allows it to transfer electrons from one compound to another. It plays a central role in cellular reduction-oxidation reactions such as the breakdown of glucose and fatty acids, allowing these substances to be utilized as energy sources[14].
Cell Signaling
In addition to its central role in metabolism, NAD+ is also involved in a crucial process known as ADP-ribosylation[15]. This process involves the addition of one or many ADP-ribose groups to proteins which have already been formed. Protein ribosylation has been demonstrated to play a role in DNA repair processes as well as modification of telomeres – both functions have well-established roles in the progression of cellular aging[16].
NAD+ has also been identified as an extracellular messenger. Preliminary research has highlighted the potential role of NAD+ in signaling between neurons, blood vessels, and even its potential role as a signaling molecule to allow for communication between nerves and the muscles they innervate[17][18].
Sirtuins
Main article: Sirtuins In 2000 it became evident that Sirtuins, a class of signaling proteins implicated in influencing cellular processes such as aging, apoptosis and inflammation were involved in transcriptional silencing. They are commonly referred to as “guardians of the genome” due to their role in regulating cellular homeostasis. Sirtuins use NAD+ to remove acetyl groups from proteins, hence they are also termed NAD-dependent deactylases e.g Sir2. Such sirtuin enzymes function by transferring an acetyl group from the substrate protein to ADP-ribose portion of NAD+. [19] Functionally, the sirtuins seem to be mainly involved in regulating transcription by deacetylating histones and consequently altering the structure of the nucleosome.
NAD+ is also utilized in ADP-ribose transfer reactions. Enzymes named ADP-ribosyltransferases add the ADP-ribose moiety to proteins creating a post-translational modification known as ADP-ribosylation. The transfer of ADP-ribose to long branched chain proteins was known as poly(ADP-ribosyl)ation. The poly(ADP-ribose) structure has gained much attention in the regulation of cellular events such as DNA repair and telomere maintenance.
In addition to intracellular functions, NAD+ has become increasingly recognized as an extracellular signaling molecule. NAD+ has been shown to be released from neurons in the blood vessels, bladder and large intestine.[20]
NAD+ Precursors
Nicotinamide Riboside
- Main article: NR
Nicotinamide Riboside (NR), often termed the ‘cousin’ of vitamin B3, is a natural substance found in trace amounts in milk, for example. NR is available to all cells and does not cause the flushing effects seen with those administering Niacin. Levels of NR do not decline in effectiveness with age, in fact it becomes more readily available during stress.
Dr Charles Brenner discovered the NR Kinase pathway in which cells use NR to create NAD+.[7] During times of stress the NR Kinase pathway increases its activity and hence cells take up NR to produce NAD+ in order to repair.
Nicotinamide mononucleotide
- Main article: NMN
Nicotinamide mononucleotide (NMN) is the immediate precursor to NAD+ in the salvage pathway of NAD+ synthesis. NMN is similar to NR in that NR becomes NMN with the addition of a phosphate group. In the past, scientists believed the addition of this phosphate on NMN makes it more difficult for NMN to enter cells and replenish NAD+ levels; however, a transporter specific for NMN, Slc12a8, has been recently identified in the gut of mice, which allows efficient transport of NMN into cells in this species. If this transporter has the same function in humans, the possibility exists for efficient transport of NMN into cells in humans. Determining how well humans can absorb NMN in comparison to NR requires further research.
Nicotinic Acid
- Main article: Niacin
Nicotinic Acid (NA) or niacin is another vitamin B3. This organic compound is an essential nutrient for humans and is commonly used to fortify packaged foods such as cereals and grains. Supplemental niacin is primarily known to be used for treating high cholesterol and pellagra.[21]
Nicotinamide
- Main article: Nicotinamide
Nicotinamide (NAM) is yet another form of vitamin B3. Also known as niacinamide, it is found in yeast, milk, meat and green vegetables. It is also used as a dietary supplement and added to foods for such benefits as treating acne [22] and reducing the risk of skin cancers.[23]
Tryptophan
- Main article: Tryptophan
Tryptophan (TRP) is an alpha-amino acid which is a vital building block in synthesizing proteins. It is also a precursor to serotonin, melatonin and vitamin B3. Tryptophans are obtained from the diet in the form of cheese, eggs and meats such as turkey.
Research
NAD+ and the process concerning its formation and depletion with age is of great importance for the future treatments of many inflammatory conditions as well as in reducing or slowing the process of aging. This fundamental biological process is associated with metabolic disorders, cancers and various neurodegenerative diseases. As research advances it is becoming more apparent that boosting NAD+ levels can be used as a potential therapeutic strategy to slowing the progression of age-related diseases such as Alzheimer’s and Parkinson’s.[24]
The differences in metabolic pathways of NAD+ biosynthesis between bacteria and humans allows for the exploitation of these differences for the development of possible new antibiotics. An example would be nicotinamidase which converts nicotinamide to nicotinic acid. This enzymes is present in bacteria but not in humans and hence is a target for drug design.[25]
NAD+ is widely sought after for supplementation, particularly in the intravenous form at various health clinics that offer treatments for those who want to improve overall health, reduce risks of cancers, or even to treat those suffering from alcohol abuse or substance misuse addictions. NAD+ supplements are readily available for purchase, particularly online.
The study of NAD+ is also implicated in the notion of a ‘biological age’ [26] as well as a chronological age. The biological age tracks the way in which our cells have changed as we have aged, this can even be specific to each organ.
The phenomenon of a biological age is a measurement based on various biomarkers and the age can change depending upon lifestyle and other health related factors. A cumulative rate of aging is the biological age relative one’s chronological age. Biological age can be a reflection of genetics, lifestyle factors and other variables such as demographics, exercise and diet.
References
- ↑ Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27(3):529‐547. doi:10.1016/j.cmet.2018.02.011
- ↑ Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5(8):593‐599. doi:10.1038/nchembio.186
- ↑ 3.0 3.1 Cell, Vol. 117, 495–502, May 14, 2004, Copyright 2004 by Cell Press Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD in Fungi and Humans
- ↑ NAD+ in Brain Aging and Neurodegenerative Disorders Lautrup, Sofie et al. Cell Metabolism, Volume 30, Issue 4, 630 – 655
- ↑ Massudi, H., Grant, R., Braidy, N., Guest, J., Farnsworth, B., & Guillemin, G. J. (2012). Age-Associated Changes In Oxidative Stress and NAD Metabolism In Human Tissue. PLoS ONE, 7(7). doi:10.1371/journal.pone.0042357
- ↑ https://www.elysiumhealth.com/en-us/science-101/everything-you-need-to-know-about-nicotinamide-adenine-dinucleotide-nad
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 https://www.aboutnad.com/announcement/1906-nad-discovered-arthur-harden-william-john-young/
- ↑ Arthur Harden and William John Young Published:24 October 1906https://doi.org/10.1098/rspb.1906.0070
- ↑ THE ISOLATION AND IDENTIFICATION OF THE ANTI-BLACK TONGUE FACTOR C. A. Elvehjem, Robert J. Madden, F. M. Strong and D. W. Woolley J. Biol. Chem. 1938, 123:137-149.
- ↑ Biochemical and Biophysical Research Communications Volume 11, Issue 1, 2 April 1963, Pages 39-43
- ↑ Imai, S., Armstrong, C., Kaeberlein, M. et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000). https://doi.org/10.1038/35001622
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/NAD%2B
- ↑ Belenky P, Bogan KL, Brenner C (2007). "NAD+ metabolism in health and disease" (PDF). Trends Biochem. Sci. 32 (1): 12–9. doi:10.1016/j.tibs.2006.11.006. PMID 17161604. Archived from the original(PDF) on 4 July 2009. Retrieved 23 December 2007.
- ↑ Cantó C, Menzies KJ, Auwerx J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31-53.
- ↑ Ziegler M. New functions of a long-known molecule: Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267(6):1550-1564.
- ↑ Bürkle A. Poly(ADP-ribose): The most elaborate metabolite of NAD+. FEBS J. 2005;272(18):4576-4589.
- ↑ Mutafova-Yambolieva VN, Sung JH, Hao X, et al. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104(41):16359-16364.
- ↑ Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of β-nicotinamide adenine dinucleotide upon stimulation of postganglionic nerve terminals in blood vessels and urinary bladder. J Biol Chem. 2004;279(47):48893-48903.
- ↑ North BJ, Verdin E (2004). "Sirtuins: Sir2-related NAD-dependent protein deacetylases". Genome Biol. 5 (5): 224. doi:10.1186/gb-2004-5-5-224. PMC 416462. PMID 15128440.
- ↑ Billington RA, Bruzzone S, De Flora A, Genazzani AA, Koch-Nolte F, Ziegler M, Zocchi E (2006). "Emerging functions of extracellular pyridine nucleotides". Mol. Med. 12 (11–12): 324–7. doi:10.2119/2006-00075.Billington. PMC 1829198. PMID 17380199.
- ↑ Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 8 October 2018. Retrieved 16 September2019.
- ↑ British National Formulary: BNF 69 (69th ed.). British Medical Association. 2015. p. 822. ISBN 978-0-85711-156-2.
- ↑ Minocha R, Damian DL, Halliday GM (January 2018). "Melanoma and nonmelanoma skin cancer chemoprevention: A role for nicotinamide?". Photodermatology, Photoimmunology & Photomedicine. 34 (1): 5–12. doi:10.1111/phpp.12328. PMID 28681504.
- ↑ Belenky P, Bogan KL, Brenner C (2007). "NAD+ metabolism in health and disease" (PDF). Trends Biochem. Sci. 32 (1): 12–9. doi:10.1016/j.tibs.2006.11.006. PMID 17161604. Archived from the original(PDF) on 4 July 2009. Retrieved 23 December 2007.
- ↑ Begley TP, Kinsland C, Mehl RA, Osterman A, Dorrestein P (2001). The biosynthesis of nicotinamide adenine dinucleotides in bacteria. Vitam. Horm. Vitamins & Hormones. 61. pp. 103–19. doi:10.1016/S0083-6729(01)61003-3. ISBN 978-0-12-709861-6. PMID 11153263.
- ↑ Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a andomized, double-blind, placebo-controlled study Ryan W. Dellinger, Santiago Roel Santos, Mark Morris, Mal Evans, Dan Alminana, Leonard Guarente and Eric Marcotulli1